Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kitamura, Akira
JAEA-Data/Code 2020-020, 164 Pages, 2021/03
Part of JAEA's Thermodynamic Database (JAEA-TDB) for solubility and speciation of radionuclides (JAEA-TDB-RN) for performance assessment of geological disposal of high-level radioactive and TRU wastes has been updated with subsuming the database for geochemical calculations (JAEA-TDB-GC). This report has focused to update JAEA-TDB-RN after selecting change in standard Gibbs free energy of formation (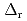
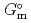 ), change in standard enthalpy change of formation (
), change in standard enthalpy change of formation (
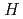

 ), standard molar entropy (
), standard molar entropy (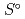
 ) and, heat capacity (
) and, heat capacity (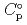 ), change in standard Gibbs free energy of reaction (
), change in standard Gibbs free energy of reaction (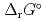
 ), change in standard enthalpy change of reaction (
), change in standard enthalpy change of reaction (
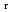
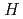

 ) and standard entropy change of reaction (
) and standard entropy change of reaction (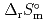 ) as well as logarithm of equilibrium constant (log
) as well as logarithm of equilibrium constant (log
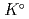 ) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
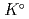 have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Kitamura, Akira
Nihon Genshiryoku Gakkai-Shi ATOMO , 62(1), p.23 - 28, 2020/01
, 62(1), p.23 - 28, 2020/01
Thermodynamic databases (TDBs) for performance assessment of geological disposal of high-level waste and TRU waste have been developed to predict solubility and speciation of radionuclides in groundwater in some countries including Japan. The present manuscript briefly describes current status of development of the TDB organized by the Nuclear Energy Agency within the Organisation of Economic Co-operation and Development (OECD/NEA) and the TDBs in some countries including Japan.
 -UO
-UO
 -CO
-CO
 system and integration with JAEA's thermodynamic database for geochemical calculations
system and integration with JAEA's thermodynamic database for geochemical calculationsKitamura, Akira
JAEA-Data/Code 2018-018, 103 Pages, 2019/03
The latest available thermodynamic data were critically reviewed and the selected values were included into the JAEA-TDB for performance assessment of geological disposal of high-level radioactive and TRU wastes. This critical review specifically addressed thermodynamic data for (1) a zirconium-hydroxide system through comparison of thermodynamic data selected by the Nuclear Energy Agency within the Organisation for Economic Co-operation and Development (OECD/NEA), (2) complexation of metal ions with isosaccharinic acid based on the latest review papers. Furthermore, the author performed (3) tentative selection of thermodynamic data on ternary complexes among alkaline-earth metal, uranyl and carbonate ions, and (4) integration with the latest version of JAEA's thermodynamic database for geochemical calculations. The internal consistency of the selected data was checked by the author. Text files of the updated and integrated thermodynamic database have been prepared for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Yui, Mikazu; ; Shibata, Masahiro
JNC TN8400 99-070, 106 Pages, 1999/11
This report is a summary of status, frozen datasets, and future tasks of the JNC thermodynamic database (JNC-TDB) for assessing performance of high-level radioactive waste in geological environments. The JNC-TDB development was carried out after the first progress report on geological disposal research in Japan (H3). In the development, thermodynamic data (equilibrium constants at 25  C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.
C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO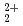 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Lothenbach, B.*; Ochs, M.*; Wanner, H.*; Yui, Mikazu
JNC TN8400 99-011, 340 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of palladium Pd, lead Pb, tin Sn, antimony Sb, niobium Nb and bismuth Bi in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system of high-level radioactive wastes. Besides treating hydrolysis in detail, this report focuses on the formation of complexes or compounds with chloride, fluoride, carbonate, nitrate, sulfate and phosphate. Other important inorganic ligands (sulfide for lead and antimony, ammonia for palladium) are also included. In this study, the specific ion interaction theory (SIT) approach is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Rai, D.*; Rao, L.*; Weger, H. T.*; GREGORY R.CHOPPI*; Yui, Mikazu
JNC TN8400 99-010, 95 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Pu(III), Am(III), and Cm(III) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(III) species are lacking, the data were selected based on chemical analogy to other trivalent actinides. In this study, the Pitzer ion-interaction model is mainly used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Rai, D.*; Rao, L.*; Weger, H. T.*; Felmy, A. R.*; Choppin, G. R.*; Yui, Mikazu
JNC TN8400 99-009, 115 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Th(IV), U(IV), Np(IV), and Pu(IV) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(IV) species was lacking, the data were selected based on chemical analogy to other tetravalent actinides. ln this study, the Pitzer ion-interaction model is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Yamato, Aiji; Sasaki, Noriaki; ; Miyahara, Kaname;
PNC TN1100 94-003, 355 Pages, 1993/11
Radioactive waste management research programs inevitably include laboratory solubili and sorption studies to provide data for radionuclide transport model. Estimation of lubility strongly depends on the reliability of thermodynamic data (e.g., carbonato-colexes) and may also depend on kinetic data on alteration of solubility limiting solid ases. Existing sorption data may include some kind of retardation mechanisms to be excded (e.g., precipitation). To develop these fundamental data, we must also consider a rge number of radioactive elements, a large number of factors (e.g., pH, Eh, complexinligands) in the repository environmentg, and numerous solid and aqueous species of radnuclides along with the many absorbents. Therefore, a systematic approach and researchlan are needed for obtaining and evaluation thermodynamic and sorption constants. The cus of this session was on thermodynamic data for aqueous species and solid phases imptant to the geological disposal system, on kinetic data